OMNIA SCIENTIA
OMNIA SCIENTIA
OMNIA SCIENTIA
OMNIA SCIENTIA
OMNIA SCIENTIA
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
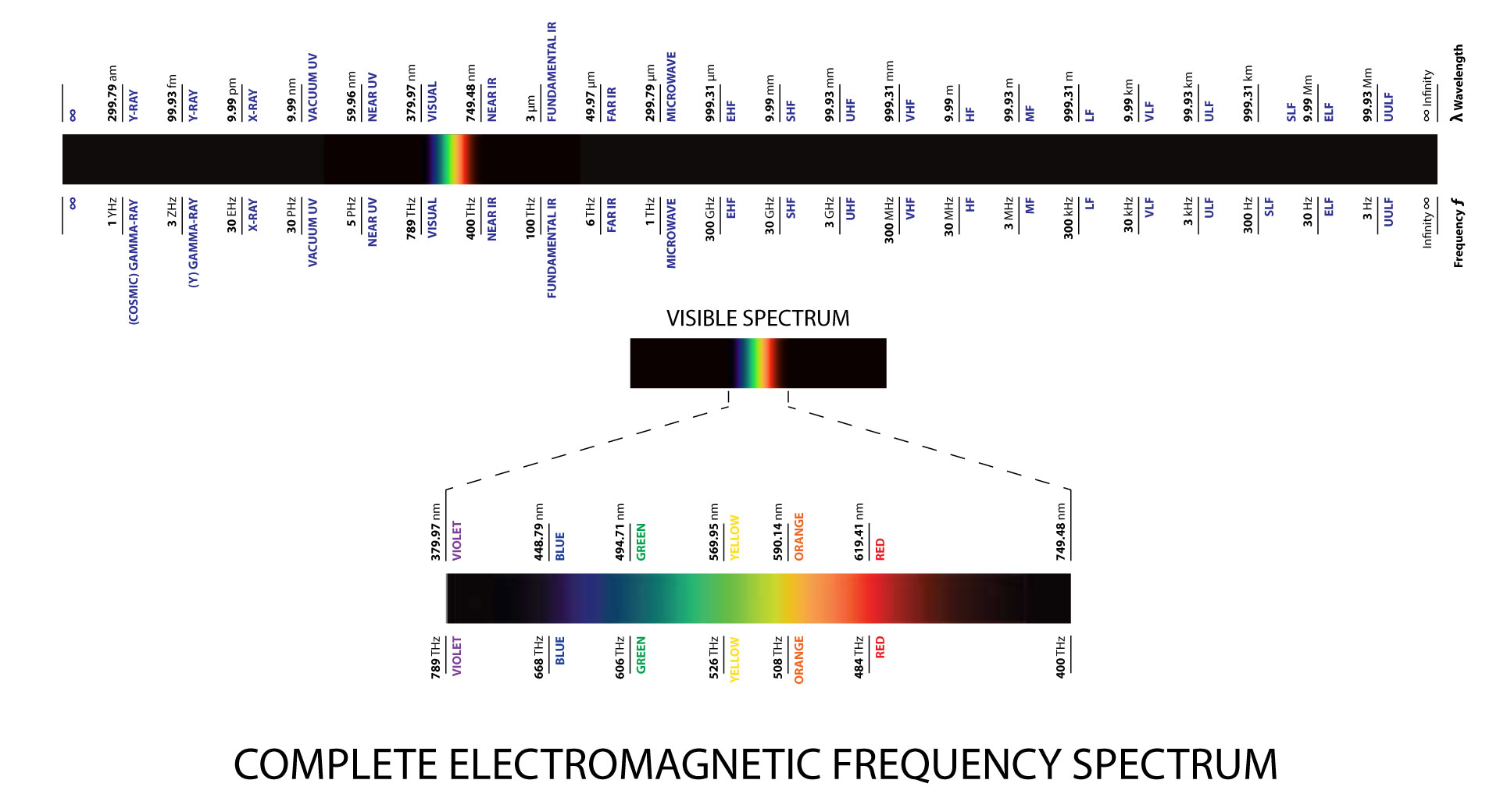
ELECTROMAGNETIC SPECTRUM
ELECTROMAGNETIC SPECTRUM
ELECTROMAGNETIC SPECTRUM
ELECTROMAGNETIC SPECTRUM
ELECTROMAGNETIC SPECTRUM
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
FREQUENCY, WAVELENGTH & ENERGY
| Electromagnetic Factors |
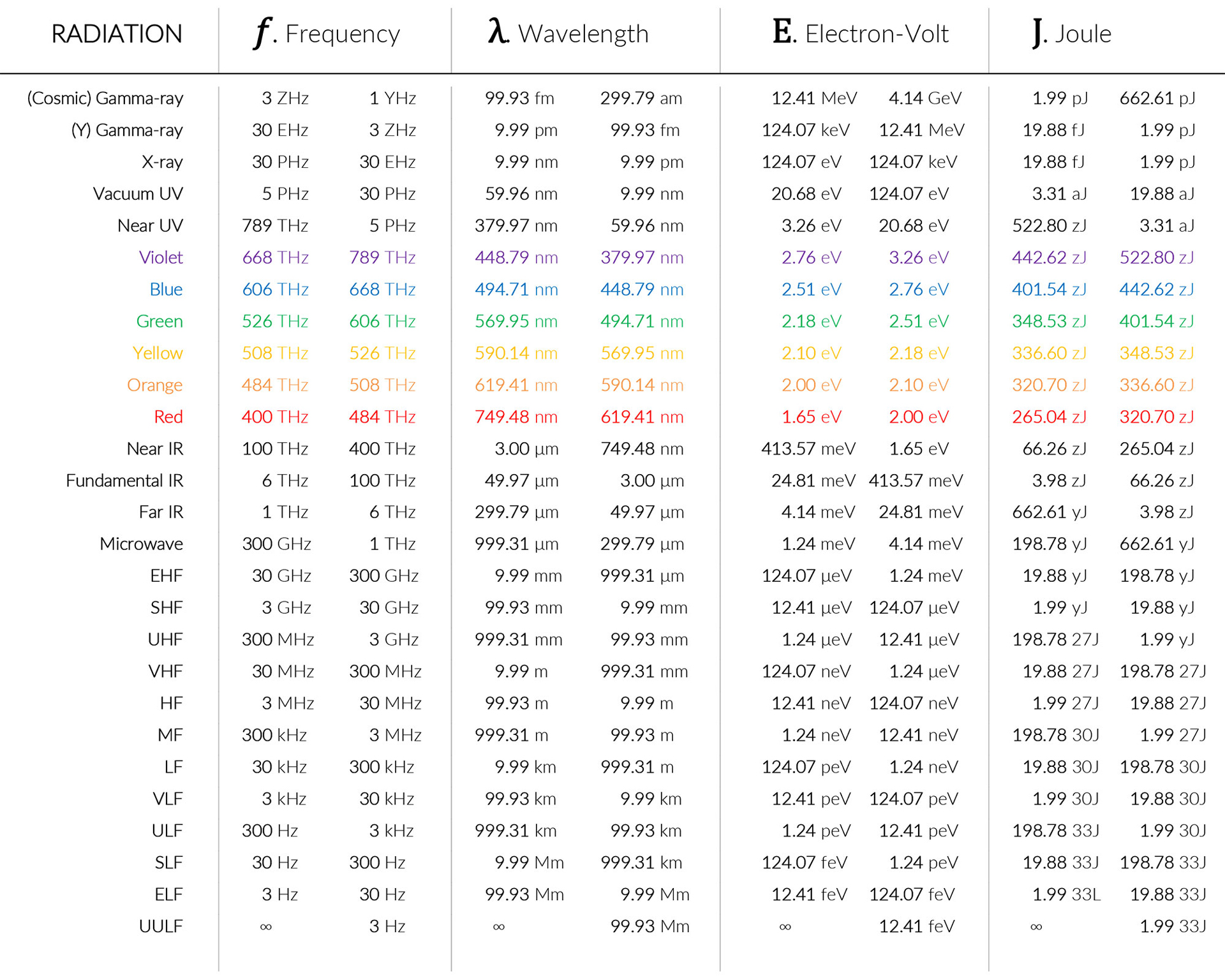
| Frequency Wavelength & Energy: One photon energy, E, of a single photon associated with the electromagnetic wave increases with frequency and is given by the relationship: E = h x f (Joules) or h x c / λ Where h is Planck’s constant at: 6.626 069 57 X 10-34 Joule seconds, or 4.13567 X 10−15 eV seconds), and f is the frequency of the wave and c is the velocity of light (299 792 458 m/s). One Electron Volt 1eV = 1.602176565 V 10-19 Joule. |
| Electromagnetic Factors |
| Frequency Wavelength & Energy: One photon energy, E, of a single photon associated with the electromagnetic wave increases with frequency and is given by the relationship: E = h x f (Joules) or h x c / λ Where h is Planck’s constant at: 6.626 069 57 X 10-34 Joule seconds, or 4.13567 X 10−15 eV seconds), and f is the frequency of the wave and c is the velocity of light (299 792 458 m/s). One Electron Volt 1eV = 1.602176565 V 10-19 Joule. |
| Electromagnetic Factors |
| Frequency Wavelength & Energy: One photon energy, E, of a single photon associated with the electromagnetic wave increases with frequency and is given by the relationship: E = h x f (Joules) or h x c / λ Where h is Planck’s constant at: 6.626 069 57 X 10-34 Joule seconds, or 4.13567 X 10−15 eV seconds), and f is the frequency of the wave and c is the velocity of light (299 792 458 m/s). One Electron Volt 1eV = 1.602176565 V 10-19 Joule. |
| Electromagnetic Factors |
| Frequency Wavelength & Energy: One photon energy, E, of a single photon associated with the electromagnetic wave increases with frequency and is given by the relationship: E = h x f (Joules) or h x c / λ Where h is Planck’s constant at: 6.626 069 57 X 10-34 Joule seconds, or 4.13567 X 10−15 eV seconds), and f is the frequency of the wave and c is the velocity of light (299 792 458 m/s). One Electron Volt 1eV = 1.602176565 V 10-19 Joule. |