NEODYMIUM, Nd (60)
HYDROGEN TO OGANESSON
ATOMIC ARCHITECTURE
NEODYMIUM
Nd (60)
ˌniːoʊˈdɪmiəm
PHONETICS
Neodymium is a soft silvery metal that tarnishes in air. It is present in significant quantities in the ore minerals monazite and bastnäsite (or bastnaesite). It is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Although neodymium is classed as a 'rare earth', it is a fairly common element, no rarer than cobalt, nickel, and copper, and is widely distributed in the Earth’s crust. Most of the world’s neodymium is mined in China.
ELEMENT BRIEF
1885 by Carl Auer von Welsbach, Austria.
DISCOVERY
Greek, 'neos didymos' meaning 'new twin'.
ETYMOLOGY
Neodymium
ELEMENT
Nd
SYMBOL
60
ATOMIC NUMBER
7440-00-8
CAS NUMBER
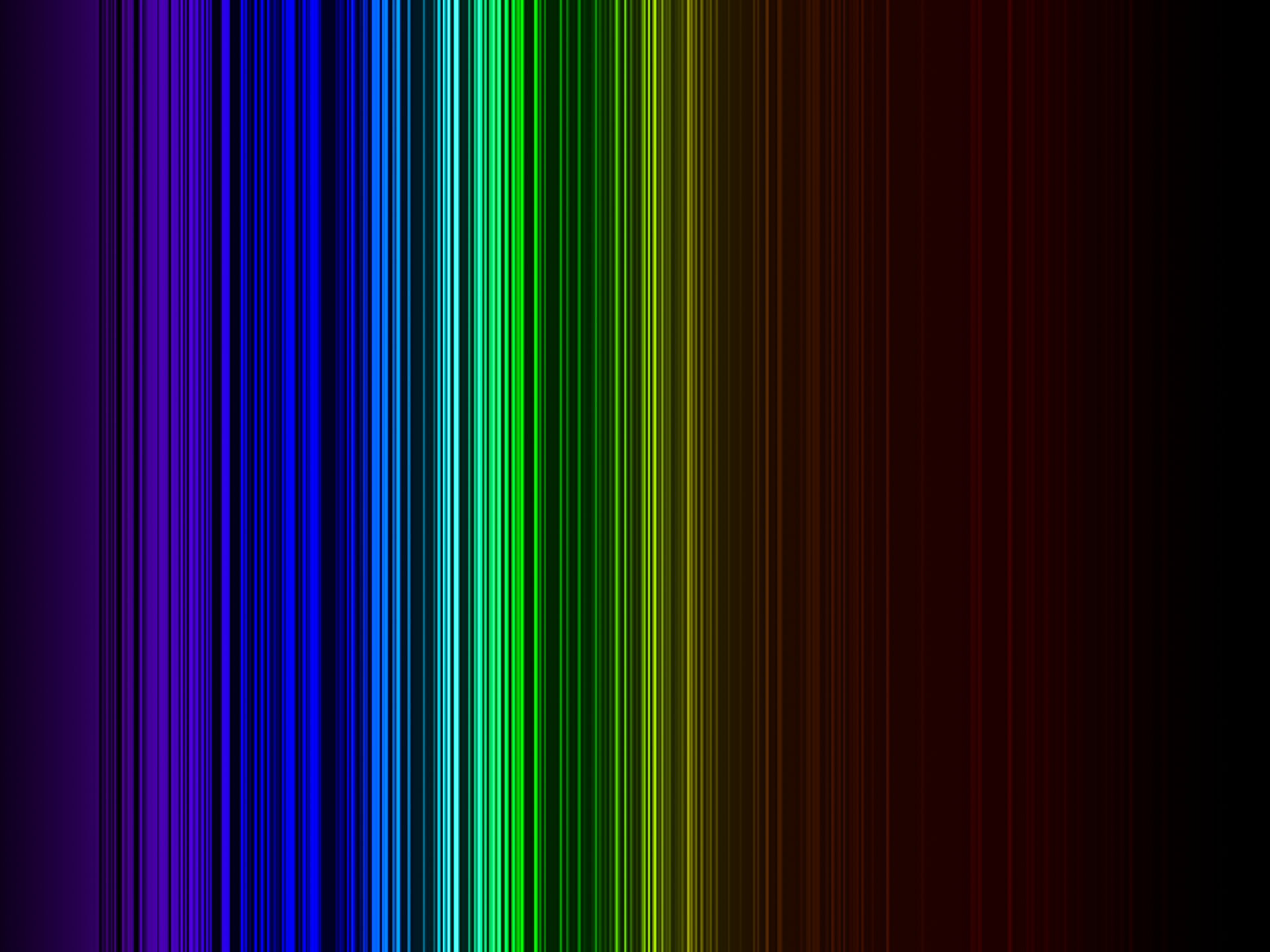
ELEMENTAL SPECTRUM
Silver
ELEMENTAL COLOUR
-
REFRACTIVE INDEX
0.280
POISSON RATIO
20.576 cm³
MOLAR VOLUME
265.00M Pa, 2.615'3k Atm
BRINELL HARDNESS
1.20
MOHS HARDNESS
343.00M Pa, 3.385'1k Atm
VICKERS HARDNESS
2,330 m/s, Mach 6.793'0
SPEED OF SOUND
32.00G Pa, 315.815'4k Atm
BULK MODULUS
16.00G Pa, 157.907'7k Atm
SHEAR MODULUS
41.00G Pa, 404.638'5k Atm
YOUNG MODULUS
ALLOTROPES
 |
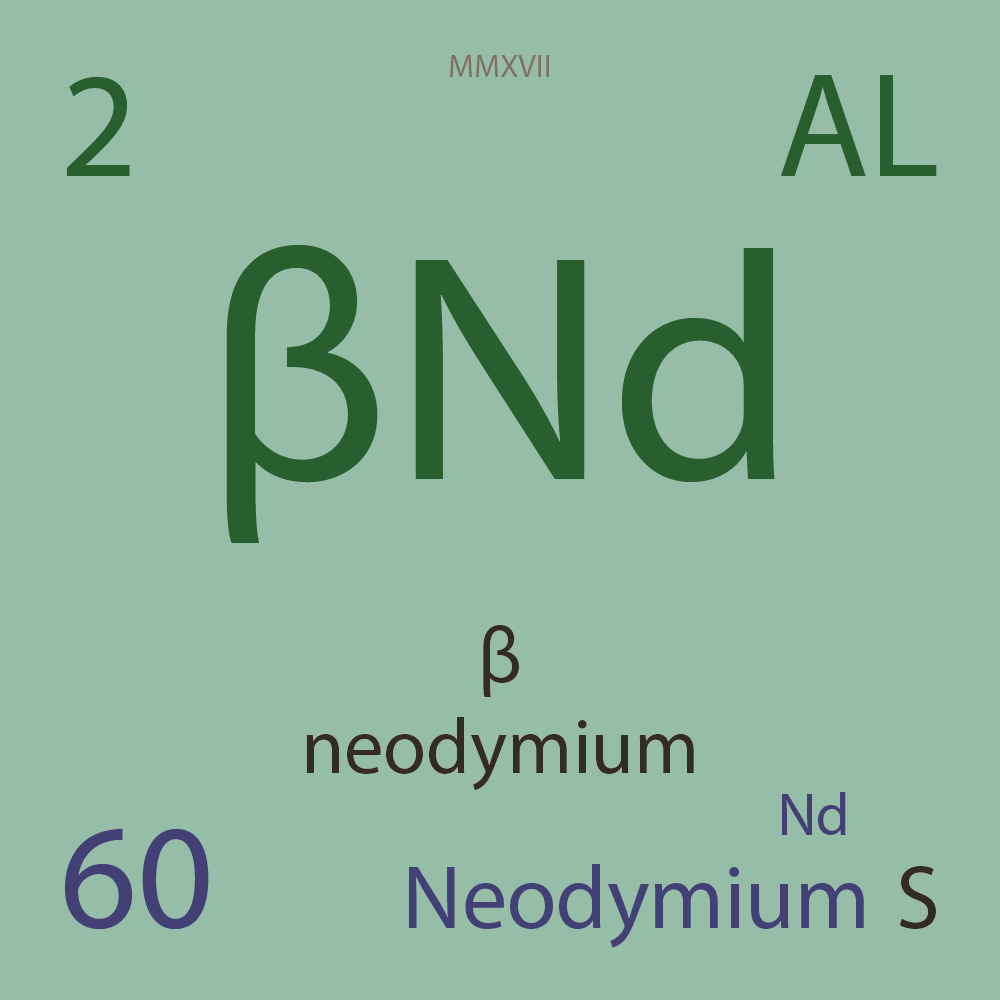 |
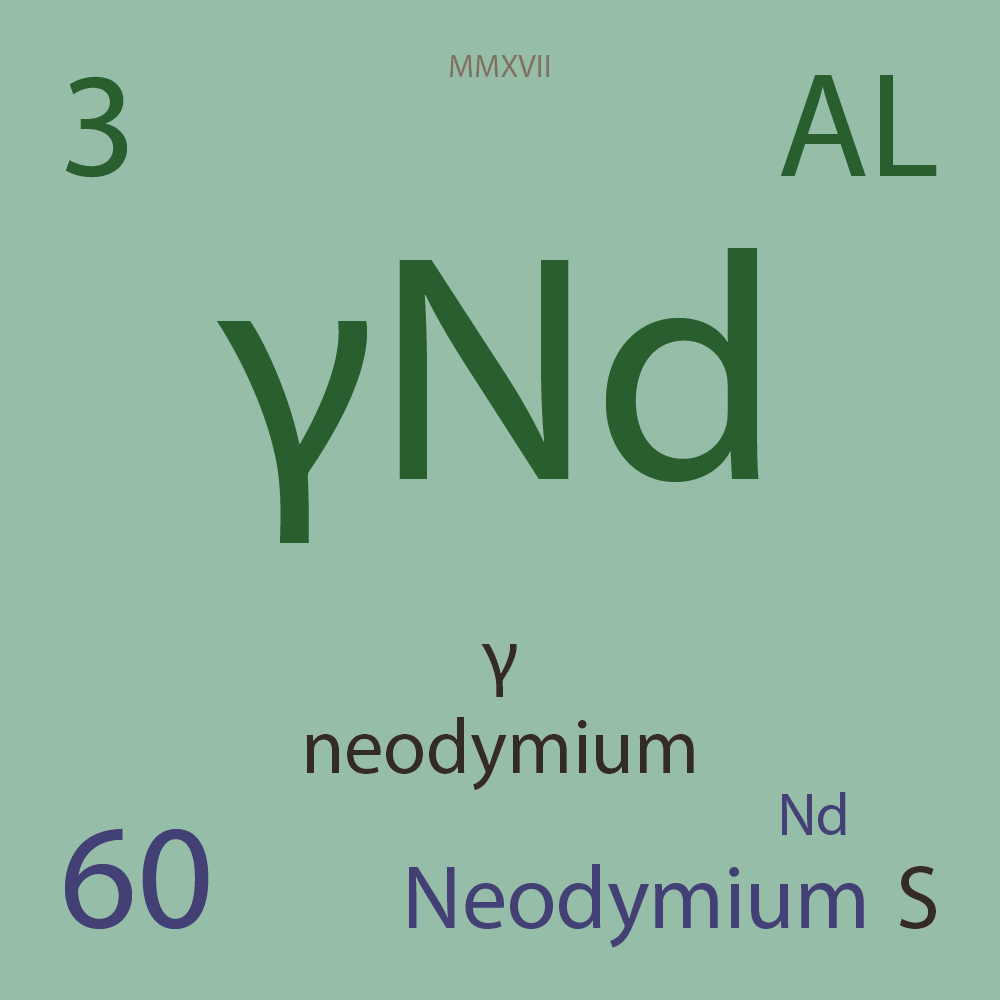 |
1,294.15 K, 1,021.00 °C
MELTING POINT
3,373.15 K, 3,100.00 °C
BOILING POINT
-
AUTOMATIC IGNITION
-
FLASHPOINT
-
CRITICAL TEMPERATURE
-
CRITICAL PRESSURE
17.000'000 W/(m K)
THERMAL CONDUCTIVITY
0.000'009'6 K¯¹
THERMAL EXPANSION
190.00 J/(kg K)
SPECIFIC HEAT
7.100 kJ/mol
HEAT FUSION
285.000 kJ/mol
HEAT VAPORISATION
-
HEAT COMBUSTION
-
CURIE POINT
19.20 K, -253.95 °C
NEEL POINT
-
ADIABATIC INDEX
Solid
PHASE
Conductor
ELECTRICAL TYPE
1.60M S/m
CONDUCTIVITY
640.00n Ω.m
RESISTIVITY
-
SUPERCONDUCTING POINT
Paramagnetic
MAGNETIC TYPE
0.000'000'480'0
MASS SUSCEPTIBILITY
0.000'000'069'235'00
MOLAR SUSCEPTIBILITY
0.003'364'800'00
VOLUME SUSCEPTIBILITY
Lanthanide
CLASSIFICATION
144.236'131'4
ATOMIC WEIGHT
206 pm
ATOMIC RADIUS
174 pm
COVALENT RADIUS SINGLE BOND
137 pm
COVALENT RADIUS DOUBLE BOND
-
COVALENT RADIUS TRIPLE BOND
229 pm
VAN DER WAALS RADIUS
[Xe]6s²4f⁴
ELECTRON CONFIGURATION
Hexagonal, Close Packed Double
CRYSTAL STRUCTURE
7.010'00 g/cm³
DENSITY AS SOLID
6.890'00 g/cm³
DENSITY AS LIQUID
-
DENSITY AS GAS
P6₃/mmc
SPACE GROUP NAME
194
SPACE GROUP NUMBER
π/2, π/2 2π/3
LATTICE ANGLES
365.8, 365.8 1179.9 pm
LATTICE CONSTANTS
3
VALENCE
1.14
ELECTRONEGATIVITY
184.87 kJ/mol
ELECTRON AFFINITY
IONISATION ENERGY
 |
 |
 |
 |
0.000'001 %
UNIVERSE
0.000'05 %
METEORITES
0.000'000'3 %
SUN
0.003'3 %
EARTH CRUST
0.000'000'000'28 %
OCEANS
-
HUMANS
Stable
HALF LIFE
Stable
LIFETIME
4f⁴ = 4, 3, 0, +1/2
QUANTUM NUMBERS
16.600'00 b σs
NEUTRON CROSS SECTION
50.500'00 b σa
NEUTRON MASS ABSORPTION
STABLE ISOTOPES
 |
 |
 |
 |
 |
UNSTABLE ISOTOPES
 |
 |
 |
 |
 |
 |
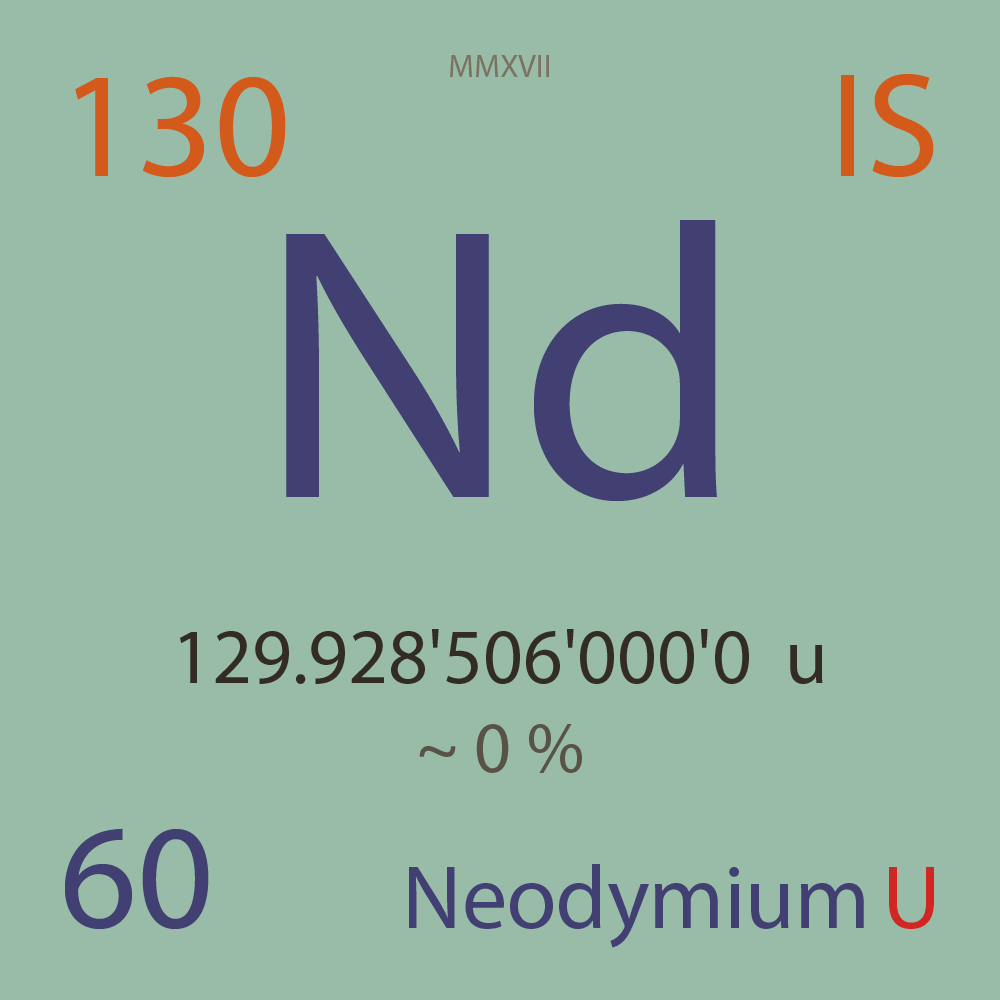 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
ISOTOPIC CHAIN
CENTRALDATACORE
SHARE
