OMNIA SCIENTIA
OMNIA SCIENTIA
OMNIA SCIENTIA
OMNIA SCIENTIA
OMNIA SCIENTIA
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
PHYSICS & CHEMISTRY
COULOMB’S LAW
COULOMB’S LAW
COULOMB’S LAW
COULOMB’S LAW
COULOMB’S LAW
FORMULA & CALCULATOR
FORMULA & CALCULATOR
FORMULA & CALCULATOR
FORMULA & CALCULATOR
FORMULA & CALCULATOR
 |
 |
 |
 |
 |
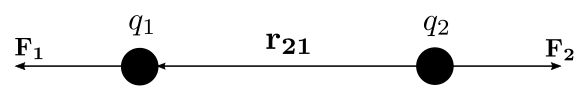
| The Coulomb or Coulomb’s inverse-square law describes the electrostatic interaction between electrically charged particles. Analogous to the inverse-square law of universal gravitation by Isaac Newton and is used to derive Gauss’s law, and vice versa. This law states: The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them. The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive. Coulomb’s law can also be stated as a simple mathematical expression. The scalar and vector forms of the mathematical equations are: |
| The Coulomb or Coulomb’s inverse-square law describes the electrostatic interaction between electrically charged particles. Analogous to the inverse-square law of universal gravitation by Isaac Newton and is used to derive Gauss’s law, and vice versa. This law states: The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them. The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive. Coulomb’s law can also be stated as a simple mathematical expression. The scalar and vector forms of the mathematical equations are: |
| The Coulomb or Coulomb’s inverse-square law describes the electrostatic interaction between electrically charged particles. Analogous to the inverse-square law of universal gravitation by Isaac Newton and is used to derive Gauss’s law, and vice versa. This law states: The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them. The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive. Coulomb’s law can also be stated as a simple mathematical expression. The scalar and vector forms of the mathematical equations are: |
| The Coulomb or Coulomb’s inverse-square law describes the electrostatic interaction between electrically charged particles. Analogous to the inverse-square law of universal gravitation by Isaac Newton and is used to derive Gauss’s law, and vice versa. This law states: The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them. The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive. Coulomb’s law can also be stated as a simple mathematical expression. The scalar and vector forms of the mathematical equations are: |
| The Coulomb or Coulomb’s inverse-square law describes the electrostatic interaction between electrically charged particles. Analogous to the inverse-square law of universal gravitation by Isaac Newton and is used to derive Gauss’s law, and vice versa. This law states: The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them. The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive. Coulomb’s law can also be stated as a simple mathematical expression. The scalar and vector forms of the mathematical equations are: |
| |F|=ke|q1q2|r2 | F1=keq1q2|r21|2ˆr21 |
| |F|=ke|q1q2|r2 | F1=keq1q2|r21|2ˆr21 |
| |F|=ke|q1q2|r2 | F1=keq1q2|r21|2ˆr21 |
| |F|=ke|q1q2|r2 | F1=keq1q2|r21|2ˆr21 |
| |F|=ke|q1q2|r2 | F1=keq1q2|r21|2ˆr21 |
| where the Coulomb’s constant (ke=8.987′551′787′368′176′4 x 109N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, the scalar r is the distance between the charges, the vector r21=r1−r2 is the vectorial distance between the charges, and ˆr21=r21/|r21| (a vector unit vector pointing from q2 to q1). The vector form of the equation calculates the force F1 applied on q1 by q2. If r21 is used instead, then the effect on q2 can be found. It can be also calculated using Newton’s third law: F2=F1. |
| where the Coulomb’s constant (ke=8.987′551′787′368′176′4 x 109N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, the scalar r is the distance between the charges, the vector r21=r1−r2 is the vectorial distance between the charges, and ˆr21=r21/|r21| (a vector unit vector pointing from q2 to q1). The vector form of the equation calculates the force F1 applied on q1 by q2. If r21 is used instead, then the effect on q2 can be found. It can be also calculated using Newton’s third law: F2=F1. |
| where the Coulomb’s constant (ke=8.987′551′787′368′176′4 x 109N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, the scalar r is the distance between the charges, the vector r21=r1−r2 is the vectorial distance between the charges, and ˆr21=r21/|r21| (a vector unit vector pointing from q2 to q1). The vector form of the equation calculates the force F1 applied on q1 by q2. If r21 is used instead, then the effect on q2 can be found. It can be also calculated using Newton’s third law: F2=F1. |
| where the Coulomb’s constant (ke=8.987′551′787′368′176′4 x 109N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, the scalar r is the distance between the charges, the vector r21=r1−r2 is the vectorial distance between the charges, and ˆr21=r21/|r21| (a vector unit vector pointing from q2 to q1). The vector form of the equation calculates the force F1 applied on q1 by q2. If r21 is used instead, then the effect on q2 can be found. It can be also calculated using Newton’s third law: F2=F1. |
| where the Coulomb’s constant (ke=8.987′551′787′368′176′4 x 109N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, the scalar r is the distance between the charges, the vector r21=r1−r2 is the vectorial distance between the charges, and ˆr21=r21/|r21| (a vector unit vector pointing from q2 to q1). The vector form of the equation calculates the force F1 applied on q1 by q2. If r21 is used instead, then the effect on q2 can be found. It can be also calculated using Newton’s third law: F2=F1. |
| Discovered in 1784 by Charles Augustin de Coulomb |
| Discovered in 1784 by Charles Augustin de Coulomb |
| Discovered in 1784 by Charles Augustin de Coulomb |
| Discovered in 1784 by Charles Augustin de Coulomb |
| Discovered in 1784 by Charles Augustin de Coulomb |
